Solco Valsartan Recall 2024 – A Robitussin recall is underway for two of the brand’s products after discovering microbial contamination of the cough syrups. Haleon (NYSE:HLN), the maker of Robitussin, notes that the recall . ConsumerAffairs is not a government agency. Companies displayed may pay us to be Authorized or when you click a link, call a number or fill a form on our site. Our content is intended to be used .
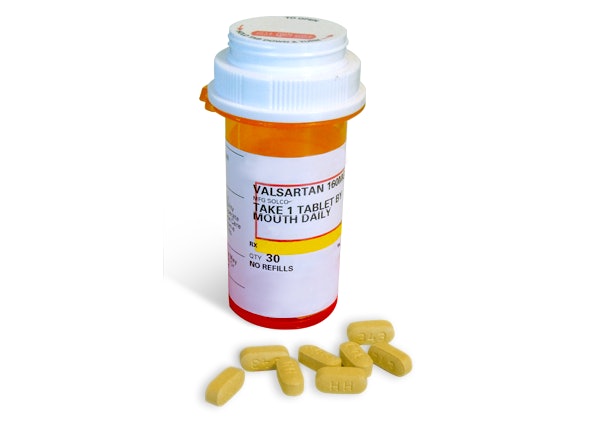
Solco Valsartan Recall 2024
Source : professionals.optumrx.comContamination Prompts Voluntary Valsartan Recall | Healthcare
Source : www.healthcarepackaging.comWhat you need to know about the Valsartan recall following cancer
Source : www.cbs42.comBlood pressure medication recall What you need to know | wwltv.com
Source : www.wwltv.comFDA: Valsartan Tablets recalled 47abc
Source : www.wmdt.comBlood Pressure Medicine Is Recalled The New York Times
Source : www.nytimes.comValsartan Lawsuit Chicago, IL Rubin & Machado Ltd.
Source : www.rubin-machado.comBlood Pressure Medicine Is Recalled The New York Times
Source : www.nytimes.comFDA joins EU in seeking recall of certain Chinese made valsartan
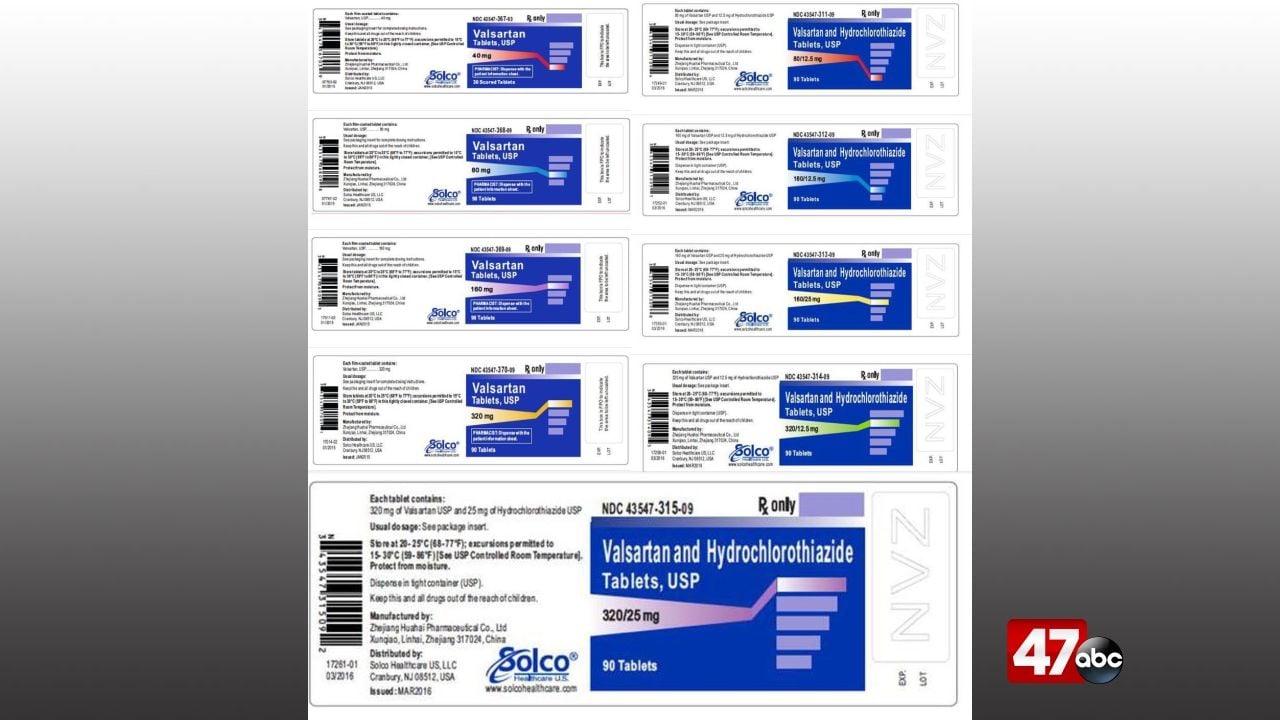
Source : www.fiercepharma.comSolco Healthcare recalls Valsartan and Valsartan HCTZ tablets
Source : www.consumeraffairs.comSolco Valsartan Recall 2024 Recall: Valsartan and Valsartan HCTZ tablets by Solco Healthcare : The initial recall in January contained only one type of cheese, but now the list comprises 22 products. A file photo of selected cheeses. The FDA has issued a wide-ranging recall of Rizo Lopez . The recall is for nearly 10 lots of its Robitussin Honey CF Max Day Adult and Honey CF Max Nighttime Adult cough syrups. “In immunocompromised individuals, the use of the affected product could .
]]>